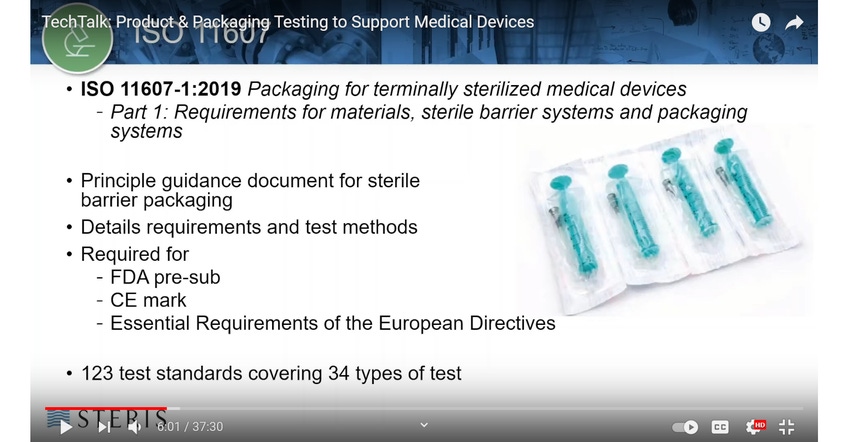
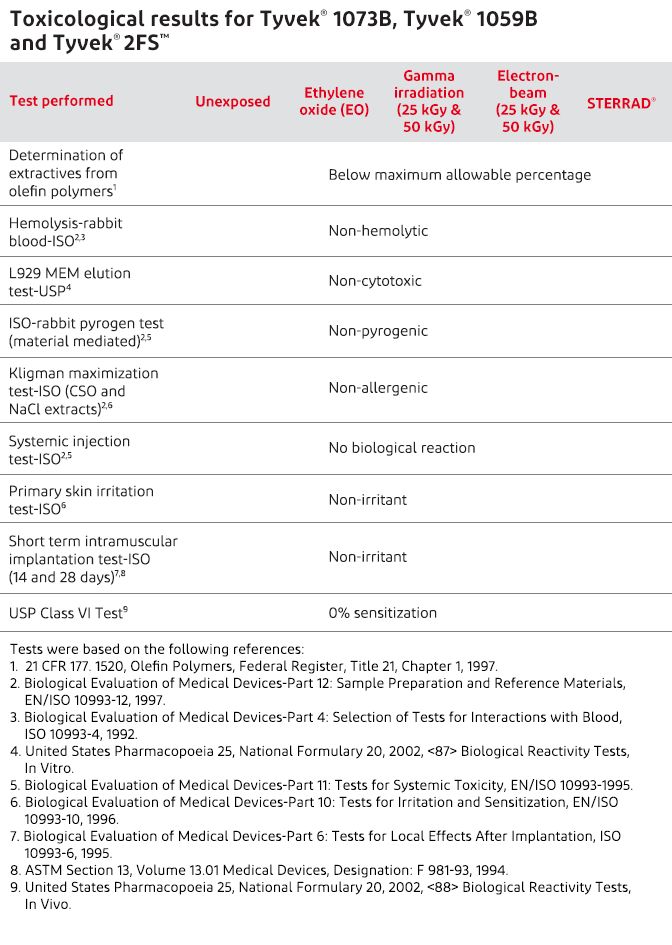
ISO 11607-1:2019/Amd 1:2023「最終段階で滅菌される医療機器の包装-第1部:材料,無菌バリアシステム及び包装システムに関する要求事項 追補1」他4点の英・日対訳版を発行しました | 一般財団法人日本規格協会のプレスリリース

EN ISO 11607-1:2020 - Packaging for terminally sterilized medical devices - Part 1: Requirements for

ГОСТ ISO 11607-1-2018: Упаковка для медицинских изделий, подлежащих финишной стерилизации. Часть 1. Требования к материалам, барьерным системам для стерилизации и упаковочным системам

ISO 11607-1:2019/Amd1:2023 - - Amendment 1: Packaging for terminally sterilized medical devices - Part 1: Requirements for materials, sterile barrier systems and packaging systems - Amendment 1: Application of risk management

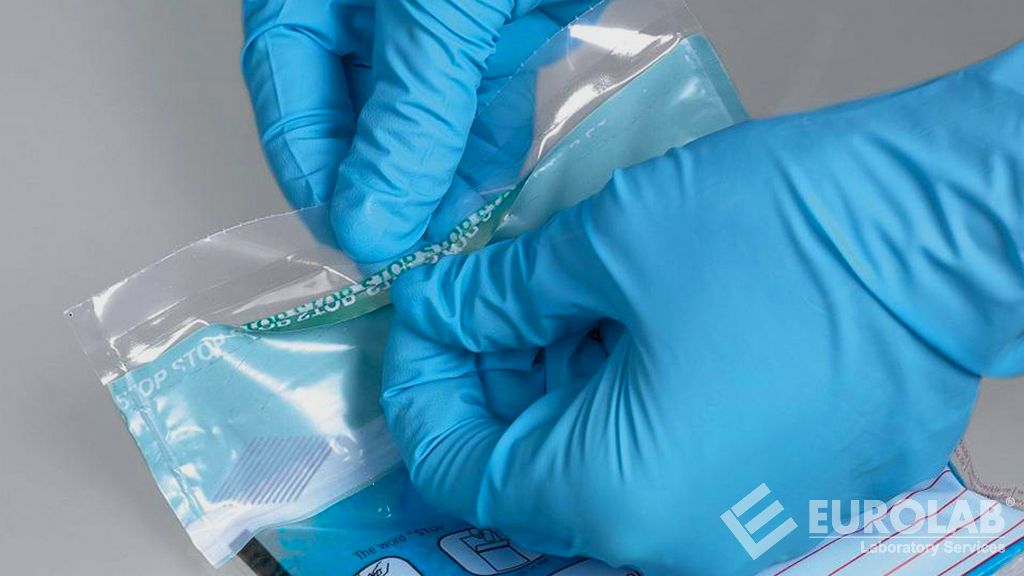
Design Terminally Sterile Packaging 3 Ways to Prevent ISO 11607 Package Design Failure (Infographic) - Life Science Outsourcing, Inc.

ISO 11607-1:2006 - Packaging for terminally sterilized medical devices - Part 1: Requirements for materials, sterile barrier systems and packaging systems

Life Science Outsourcing, Inc. on LinkedIn: #medicalexcellence #iso11607 #lifescienceoutsourcing #medicalpackagetesting

BS EN ISO 11607-1:2009 - Packaging for terminally sterilized medical devices. Requirements for materials, sterile barrier systems and packaging systems (British Standard)